BRC BIOTECHNOLOGY
BRC Biotechnology has built comprehensive development and validation capabilities across molecular, microbial, viral, cell, and animal technology platforms, in strict compliance with global pharmacopoeias, regulations, and industry quality standards. Adhering to these rigorous industry quality standards, BRC Biotechnology has achieved a series of prestigious certifications, including the EU GMP Qualified Person (QP) compliance statement, the United States FDA Facility Establishment Identifier (FEI), ISO 9001: 2015 certification, Biosafety Level 2 (BSL-2) accreditation, China Metrology Accreditation (CMA), China National Accreditation Service for Conformity Assessment (CNAS), and more.
With years of biosafety testing experience, deep regulatory expertise, and strong innovation capabilities, BRC Biotechnology has delivered high-quality, compliance-driven biosafety testing services to dozens of domestic and international biotech companies. Our services have been instrumental in supporting their products through the regulatory approval processes with the National Medical Products Administration (NMPA), the European Medicines Agency (EMA), the U.S. Food and Drug Administration (FDA), and other regulatory bodies for Investigational New Drug (IND) and Biologics License Application (BLA) submissions.
As your reliable biotech-service partner on the path to success, we are committed to delivering world-class biosafety services to ensure your products meet the highest standards of quality and safety.
Engaged Clients
100+
Delivered Tests
6000+
FDA/EMA Submission Support
20+
Delivered Projects
600+
Commercial Projects
40+
BLA Projects
60+

Comprehensive
Capability Across
Multiple
Modalities
Cell and Gene Therapy
Viral Vector, Oncolytic Virus, Stem Cell,
CAR-T/TCR-T, CAR-NK, TIL, etc
Recombinant Protein
Growth Factor, Insulin, etc
Vaccine
Attenuated, Inactive, Subunit Vaccine
Nuclear Acid Drug
mRNA, siRNA, etc
BRC Biotech’s Compliant
Quality System Ensures
Regulatory Adherence
A rigorous quality system is the foundation of compliance-based biosafety assurance. BRC Biotechnology strictly adheres to the requirements of domestic and international regulatory systems, has established a quality system that complies with the GMP standards of China, the USA, and Europe, ensuring quality from the source by meticulously selecting GMP-grade raw materials and adhering to GMP standards, consistently upholding the highest global quality standards. BRC Biotechnology fully welcomes regular customer audits and relies on a comprehensive quality compliance system and strong research capabilities to provide clients with professional risk assessments and customized solutions.
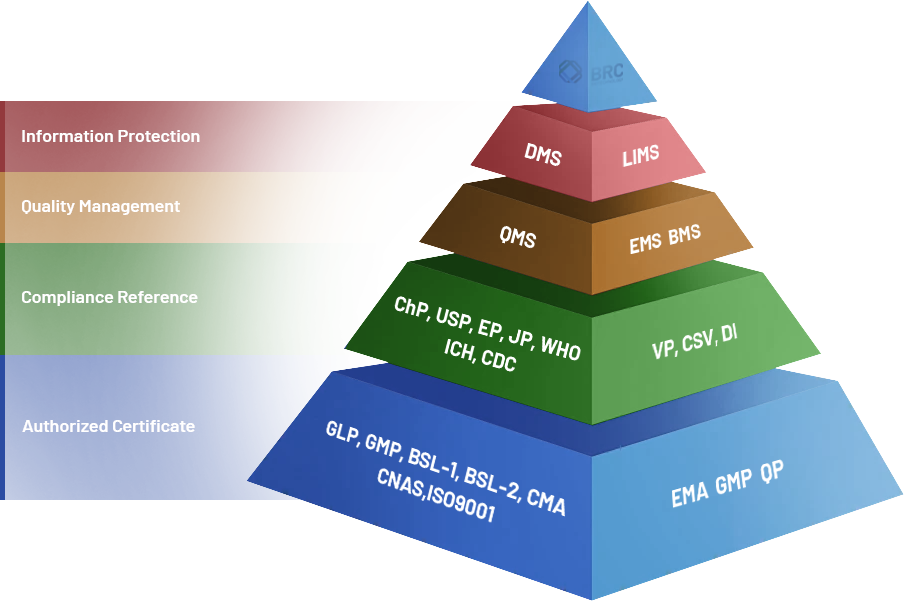
The Certifications We Have Obtained:


EU GMP Qualified Person (QP) compliance statement


United States FDA Facility Establishment Identifier (FEI)


ISO 9001: 2015


EU GMP Qualified Person (QP) compliance statement


EU GMP Qualified Person (QP) compliance statement


EU GMP Qualified Person (QP) compliance statement
One-Stop Solution for Biosafety
with In-House Capabilities
with In-House Capabilities