
ICH Q5A (R2), WHO, EMA, FDA, and NMPA encourage the adoption of Next Generation Sequencing (NGS) as a supplement or alternative to traditional viral detection methods. Because of the sensitivity and scope of virus detection, NGS has been increasingly applied in viral safety evaluation of biologics products
BRC Biotechnology's patented NGenius® Testing Platform leverages NGS technology to deliver compliant, efficient, sensitive, and accurate adventitious virus detection services, ensuring biologics safety and accelerating time-to-market.
NGenius® T Testing Platform enables accurate and reliable viral genome identification through proprietary target-capture probe design.
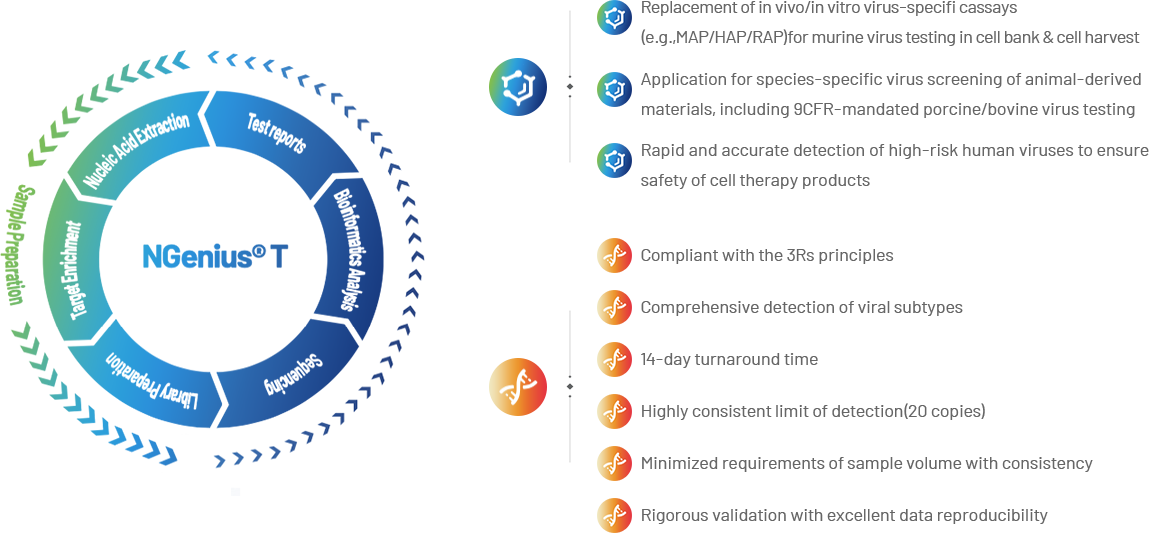
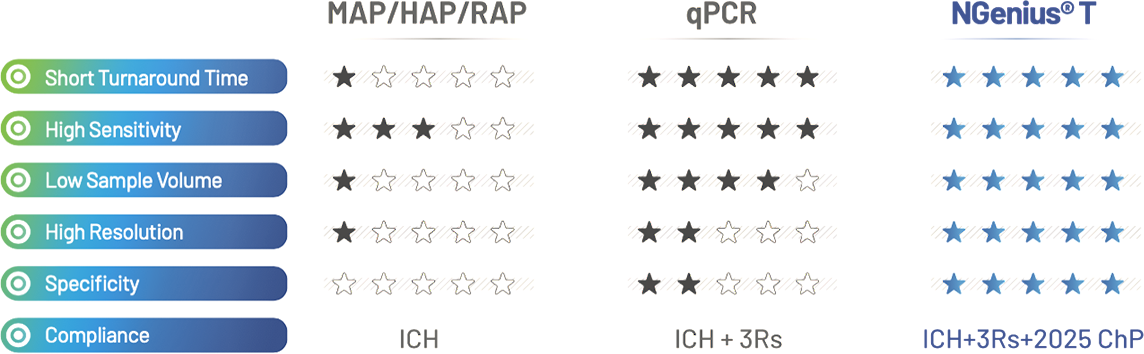
As a replacement of in vivo/in vitro virus-specific assays, comparing to other methods, NGenius® platform offers comprehensive advantages in different aspects.
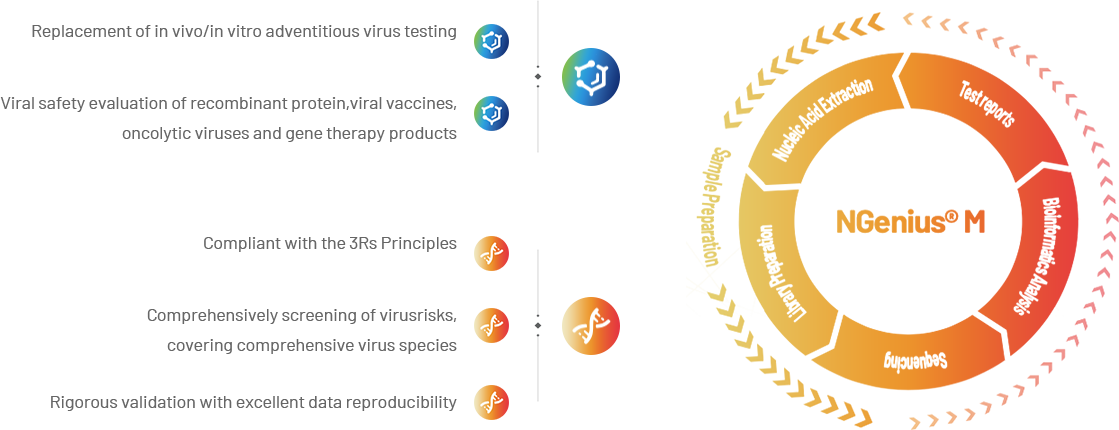
NGenius® M Testing Platform is a rapid and efficient adventitious virus screening platform powered by metagenomic sequencing technology.
We committed to providing professional compliance biosafety testing service, assay development services and testing products for cell & gene therapy, antibodies, vaccines, nucleic acid drugs and other biological products. As your reliable biotech-service partner on the way to success, we are committed to providing world-class the biosafety services for you.
Contact us for more information of NGenius® Testing Platform.