One-Stop Solution for Biosafety with In-House Capabilities
BRC Biotechnology's Detection Platform is equipped with biosafety testing capabilities that integrate GMP standards, spanning a wide range of specialized domains, including molecular biology, microbiology, virology, cell biology, animal studies, immunology, and viral clearance. The platform adheres strictly to GMP, GLP, ISO, CNAS, and CMA standards, as well as Biosafety Level 2 (BSL-2) requirements. BRC Laboratories utilizes state-of-the-art equipment from leading multinational manufacturers, ensuring rigorous quality control and validation to meet 21 CFR Part 11 requirements for electronic signatures and data integrity. BRC Biotechnology has a proven track record, having passed audits from more than 100 global clients and successfully delivered over 100 IND/BLA submission projects to date.
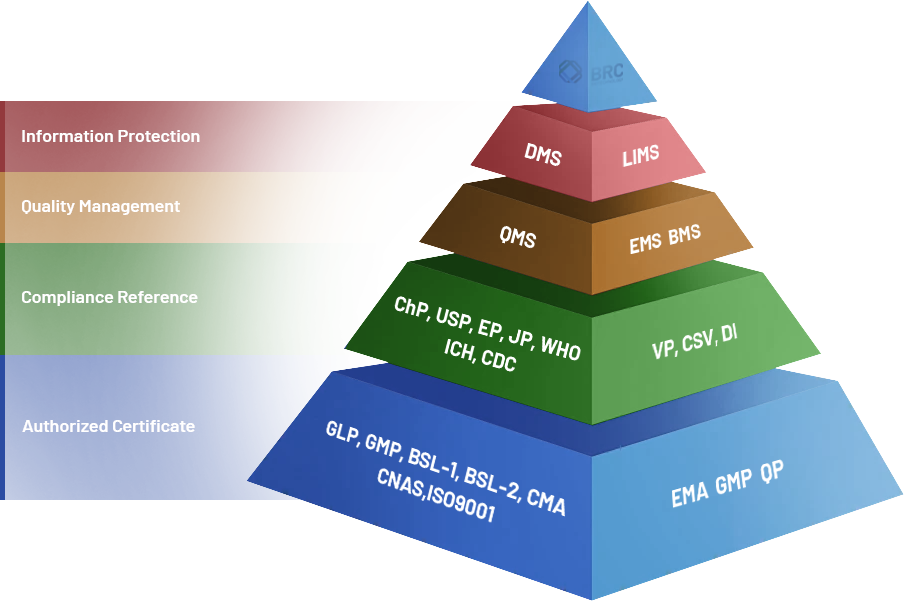
EU GMP Qualified Person (QP) compliance statement
United States FDA Facility Establishment Identifier (FEI)
ISO 9001: 2015
Biosafety Level 2 (BSL-2)
China Metrology Accreditation (CMA)
China National Accreditation Service for Conformity Assessment (CNAS)