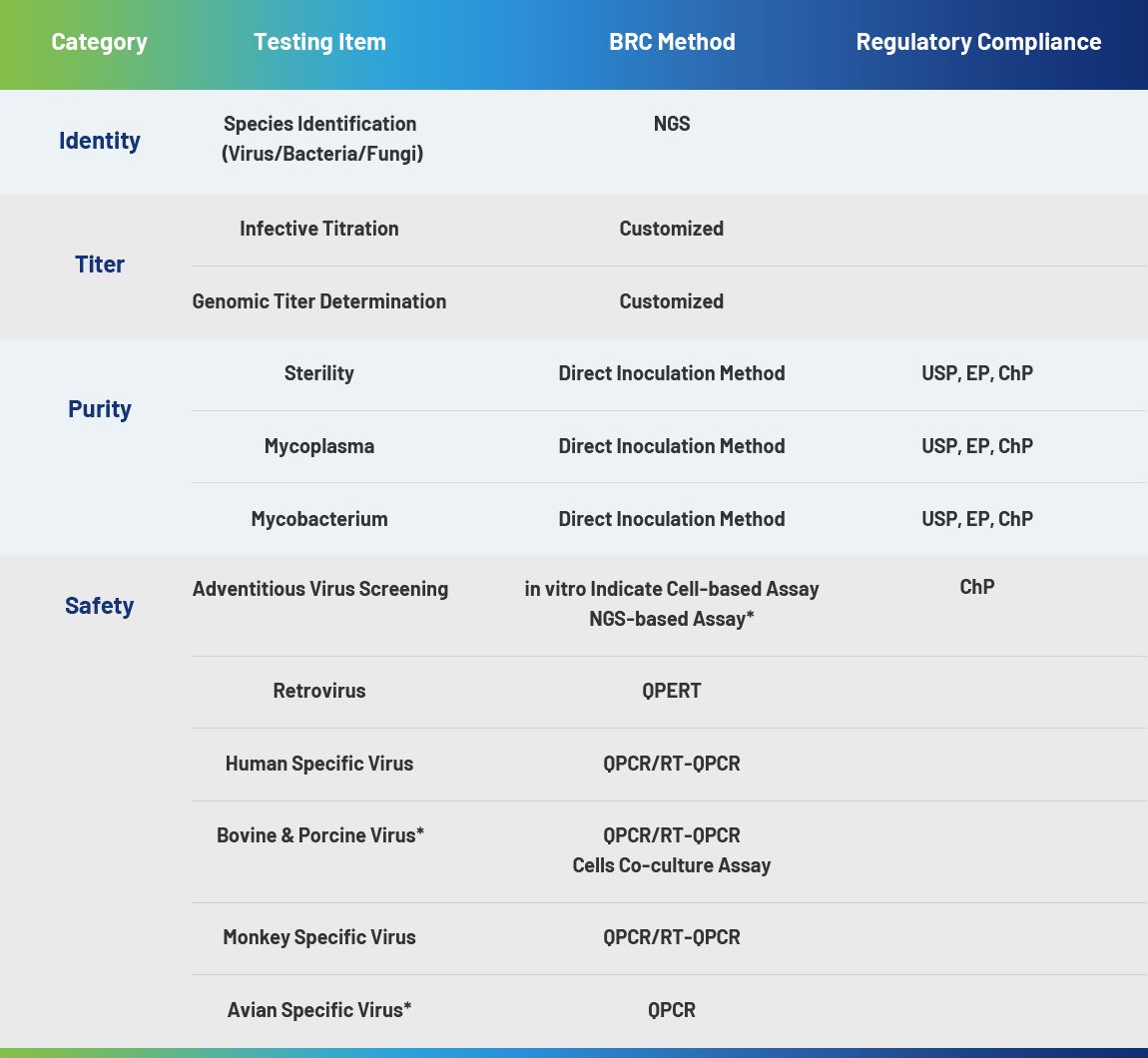
Recombinant protein therapies – including major biologics like monoclonal antibodies – are produced by inserting target DNA into bacterial or mammalian host cells, expressing the encoded proteins, and purifying them from cellular systems. Widely used as host, Escherichia coli (E. coli) plays a key role in recombinant protein production. There have been various approved therapeutic proteins produced in E. coli, contributing to the low cost. Despite these advantages, the production process is complex and requires strict control over key indicators such as host residuals and process impurities. BRC Bio offers quality testing for E. coli and plasmid products, meeting the requirement of gene therapy, gene-modified cell therapy, vaccine, nuclear acid drug etc.