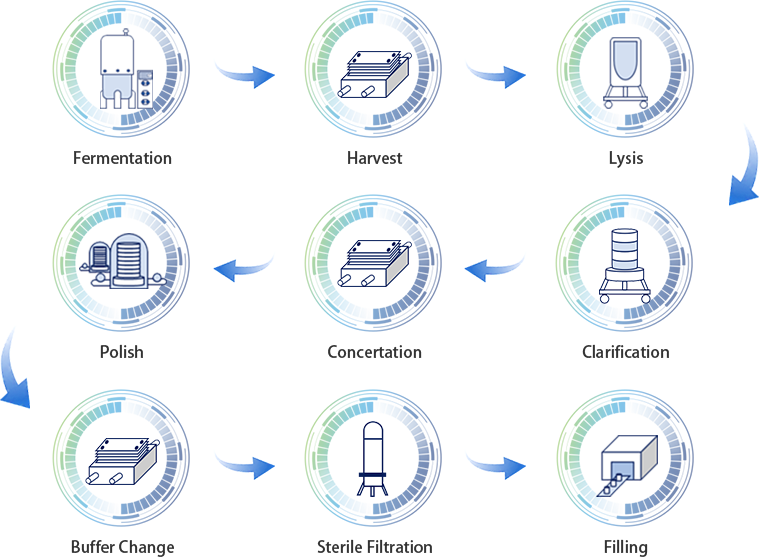
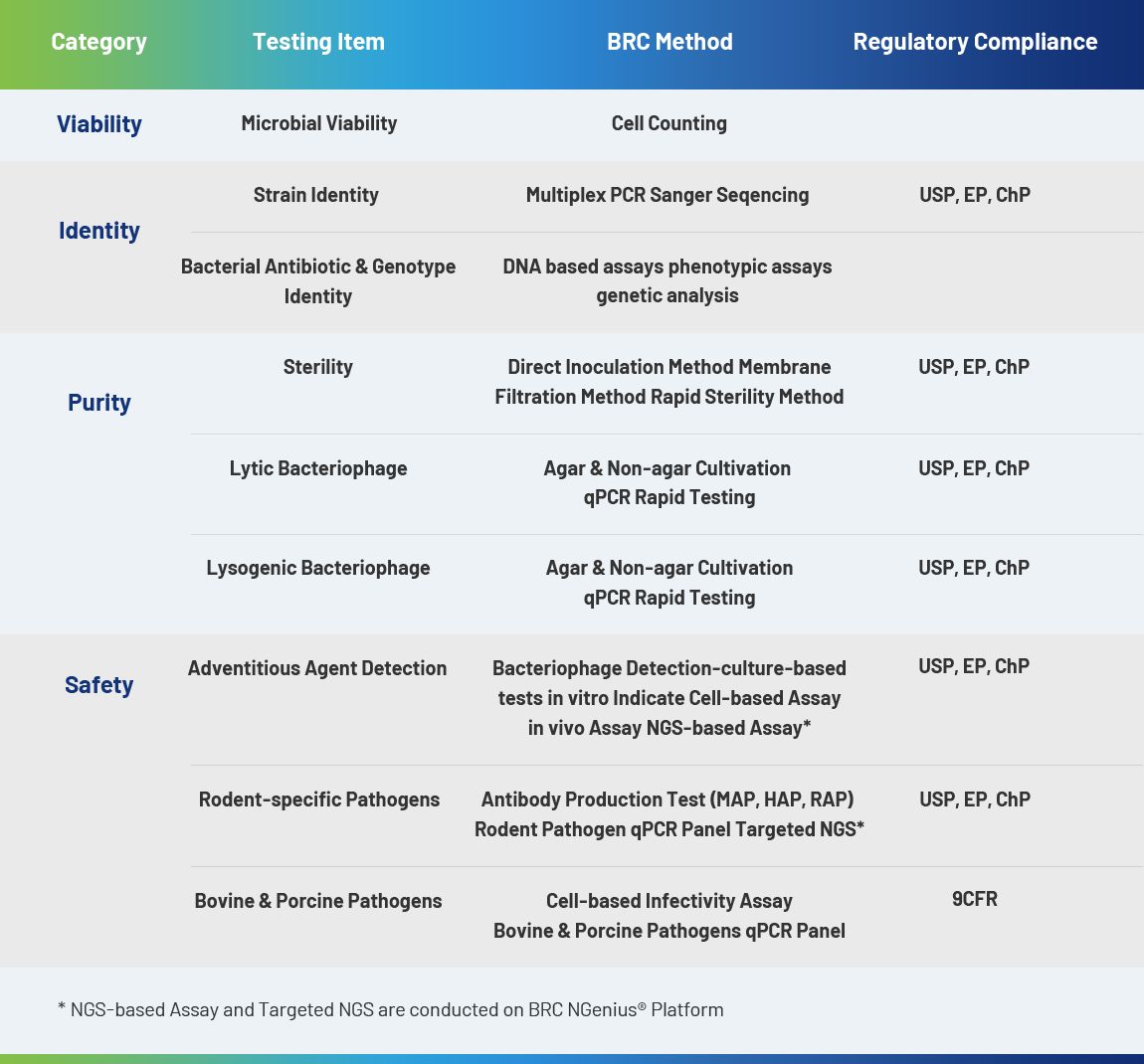
E. coli and its plasmids play an important role in biopharmaceuticals, which are used in the research, development and production of different biological modalities to meet the requirement of large-scale clinical applications.



Purityof Microbial Bank

Plasmid Retention Ratein HostStra

Antibiotic Residual Testing

Lysis Efficiency of Bacteria

Endotoxin Residual

Plasmid Construction

Genetic Stability of E.coli

Bacteriophage Contamination

......