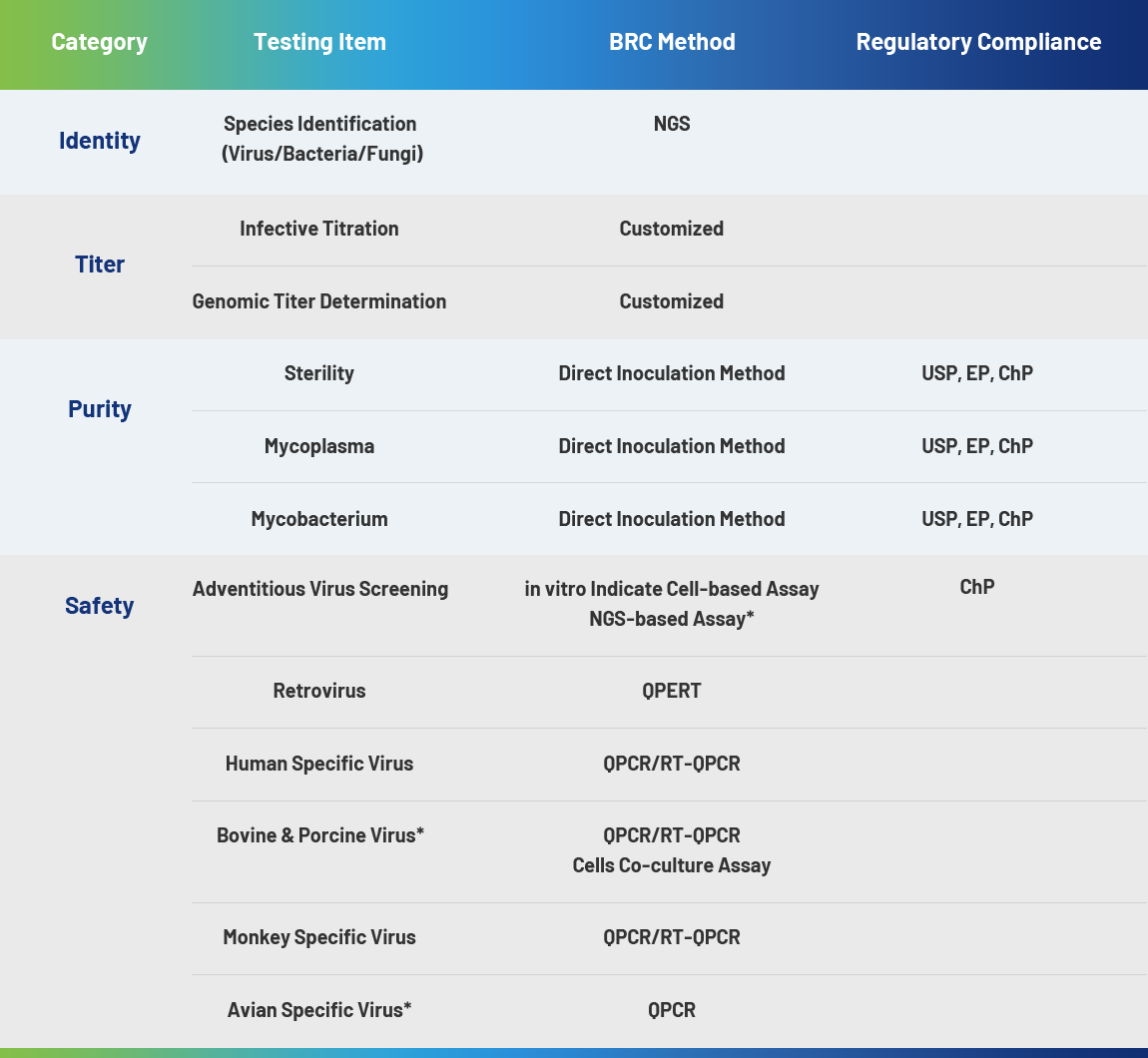
Cell and gene therapies (CGT) are advanced biological products including viral vectors, oncolytic viruses, stem cells, CAR-T/TCR-T, CAR-NK, and tumor-infiltrating lymphocytes (TILs). Due to their unique mode of action (MOA), the manufacturing processes of these emerging biologics are more complex compared to traditional biologics and small molecule therapeutics. They require stringent GMP controls while experiencing rapid market growth. To ensure final product quality, developing science-driven, process-informed biosafety testing strategies that meet global regulatory standards is essential.