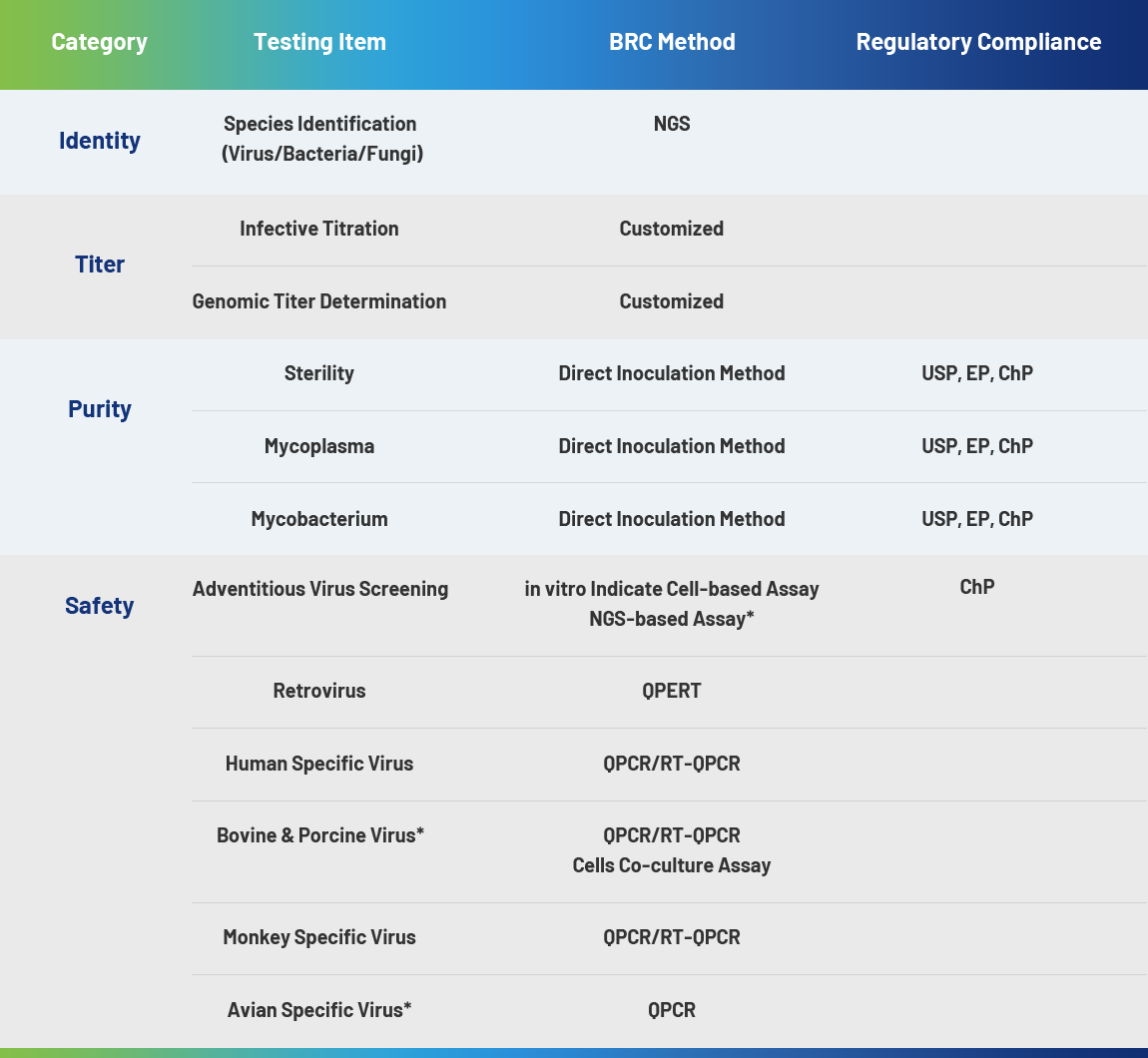
Vaccines provide active acquired immunity to diseases. Since vaccines are directly given to healthy populations, R&D and production of vaccines demand extremely stringent biosafety requirements. A closed-loop quality control system spanning the entire workflow- from raw material control and process monitoring to product release- must be established to comprehensively reduce biosafety risks.